Indonesia’s medical device market is growing fast, creating significant opportunities for Chinese manufacturers. However, entering this market is not easy. This is where consulting firms become essential, providing a clear path and expert guidance to help Chinese businesses navigate the end-to-end import procedure with confidence.
So, how can consulting firms streamline medical device imports in Indonesia? Explore more here and learn how to navigate the challenges seamlessly.
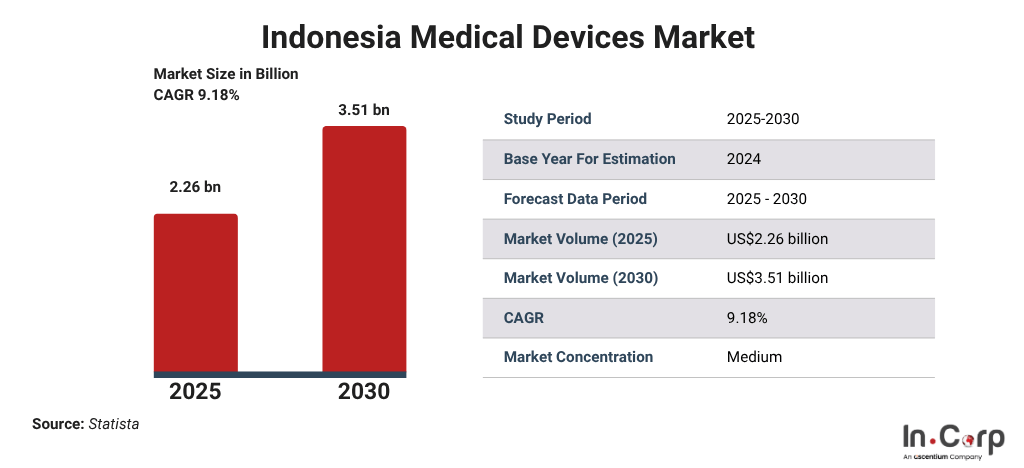
The growth of Indonesia’s medical device market
Indonesia is currently one of Southeast Asia’s fastest-growing markets for medical devices. The revenue in this sector is expected to reach US$2.26 billion in 2025 and grow annually by 9.18% (CAGR 2024–2030), with an estimated value of US$3.51 billion by 2030. Several factors are accelerating this growth:
- An aging population is increasing the demand for diagnostic and therapeutic equipment.
- Private hospital growth is boosting the procurement of imported medical devices.
- Increasing adoption and customer preferences of digital health technologies (wearable devices, remote patient monitoring systems, and telemedicine).
- Rising disposable income levels affect the increasing healthcare spending.
This rapid expansion opens new doors for foreign manufacturers, especially from China. However, entering the market requires an innovative and compliant strategy.
Read more: How to establish medical device manufacturing company in Central Java
Key opportunities for Chinese businesses
If you want to expand your medical device business to Indonesia, this is the right time. Here is what Indonesia has to offer for Chinese companies to consider:
Growing Demand & Competitive Advantage
Indonesia’s large population and growing healthcare needs are driving demand for modern medical devices. With longer life expectancy and more chronic health issues, the need for reliable medical technology is rising fast.
However, local production only covers 10–15% of this demand, meaning 85–90% of devices are imported. This creates a significant opportunity for Chinese manufacturers. Key advantages for Chinese businesses include:
- Low-cost production
- Scalable operations
- A wide range of products
- Strong global trade experience
With the right strategy, they can offer quality devices at lower prices than many Western brands.
High Dependence on Imports
Indonesia remains highly dependent on imported medical devices, especially for advanced categories such as diagnostic imaging, ICU equipment, and surgical instruments. By navigating the local bureaucracy, Chinese businesses can gain quick traction in this space.
Read more: Mastering import regulations in Indonesia: Restructuring for success
Strategic challenges Chinese business will encounter
While the opportunities are strong, entering Indonesia’s medical device market is not without its challenges. Chinese companies must navigate these common pitfalls, including:
Regulatory Complexity & Localization
Regulatory compliance is the biggest hurdle for Chinese companies, particularly around localization policies. To sell medical devices in Indonesia, businesses must:
- Register their products with the Ministry of Health (MoH)
- Appoint a local distributor or license holder
- Meet local content requirements (TKDN) in some device categories
- Translate technical documents into Bahasa Indonesia
These requirements vary depending on the device’s risk classification and local innovation level.
Business Licensing & Importation Barriers
Chinese manufacturers must also secure:
- A business license for operating in Indonesia
- An import permit (API-U or API-P) depending on business structure
- Customs clearance and conformity assessments for each shipment
Without local knowledge, these steps can become time-consuming. This is where consulting firms play a critical role in ensuring compliance.
Market Competition & Cultural Barriers
Another challenge is understanding local market dynamics. Indonesia’s healthcare system is diverse, with a mix of public and private buyers. Cultural nuances also affect business negotiations, branding, and distribution strategy. By gaining a deep understanding of these factors, Chinese businesses can enter the market prepared and knowledgeable.
Read more: Navigating the landscape of Indonesian imports
How consulting firms can bridge the gaps
Consulting firms make it much easier for Chinese businesses to enter the Indonesian medical device market. This is how they can help to reduce stress and time-consuming process:
1. Help with Product Registration
Consulting firms know precisely how to handle Indonesia’s product registration process. They help with:
- Document preparation and translation
- Products standardization following Indonesia’s health regulations.
- Paperwork submission to the Ministry of Health
- Correct device classification to speed up approval
2. Import Permits and Licenses Support
To import and sell in Indonesia, you need the proper permits. Consulting firms assist with:
- Getting the proper import license (API-U or API-P)
- Applying for a business license (NIB)
- Managing customs paperwork and legal setup
This helps avoid delays at the port or rejections from local authorities.
3. Local Partner and Market Access
Foreign companies need a local partner to register products. Consulting firms can:
- Act as your official local representative
- Connect you with trusted distributors and agents
- Offer market research to help you set prices and reach hospitals
With the help of consulting firms, Chinese businesses can launch faster, avoid mistakes, and grow with confidence in the Indonesian market.
Read more: Key criteria when choosing a business consulting firm
Clear Paths to Product Registration in Indonesia

Streamline your importation with InCorp
Indonesia offers substantial potential for Chinese medical device companies—but only if they understand and meet the country’s strict regulations. InCorp Indonesia (an Ascentium Company) can help Chinese businesses tap into these opportunities.
With deep knowledge of local laws and procedures, our experts help businesses:
- Secure the proper business license for operating in Indonesia
- Handle end-to-end medical devices registration with the Ministry of Health
- Apply for the correct import permits to bring medical devices into the country
Whether you are a startup or a large-scale manufacturer, InCorp ensures a smoother, faster, and fully compliant market entry.
Ready to enter Indonesia’s booming medical device market? Contact us today and start your expansion with confidence.
Frequently Asked Questions
Why is Indonesia an attractive market for Chinese medical device companies?
Indonesia’s growing population and healthcare needs create high demand for modern medical devices—85–90% of which are imported, giving Chinese manufacturers a strong entry opportunity.
What are the key advantages for Chinese medical device businesses?
Chinese companies benefit from low-cost production, scalable operations, diverse product lines, and strong international trade experience—allowing them to compete effectively with Western brands.
What are the main challenges Chinese businesses face in Indonesia?
The biggest hurdles include complex regulations, localization requirements (TKDN), licensing barriers, and navigating cultural and market differences.
What licenses and permits are required to sell medical devices in Indonesia?
Companies must obtain a business license (NIB), import permit (API-U or API-P), and register products with the Ministry of Health before distribution.
How can consulting firms like InCorp help Chinese companies enter Indonesia?
InCorp assists with product registration, import licensing, local representation, and market access to ensure fast, compliant, and successful expansion into Indonesia’s medical device industry.
Get in touch with us.
What you'll get
A prompt response to your inquiry
Knowledge for doing business from local experts
Ongoing support for your business
Disclaimer
The information is provided by PT. Cekindo Business International (“InCorp Indonesia/ we”) for general purpose only and we make no representations or warranties of any kind.
We do not act as an authorized government or non-government provider for official documents and services, which is issued by the Government of the Republic of Indonesia or its appointed officials. We do not promote any official government document or services of the Government of the Republic of Indonesia, including but not limited to, business identifiers, health and welfare assistance programs and benefits, unclaimed tax rebate, electronic travel visa and authorization, passports in this website.