The Indonesian Food and Drug Authority, or Badan Pengawas Obat dan Makanan (BPOM), plays a crucial role in safeguarding the country’s food, drugs, and cosmetics safety and quality. BPOM is also responsible for other regulated products entering and circulating within the Indonesian market. As a business looking to import or manufacture these products in Indonesia, obtaining permits from BPOM in Indonesia is crucial for your operation.
Understanding the BPOM certification in Indonesia
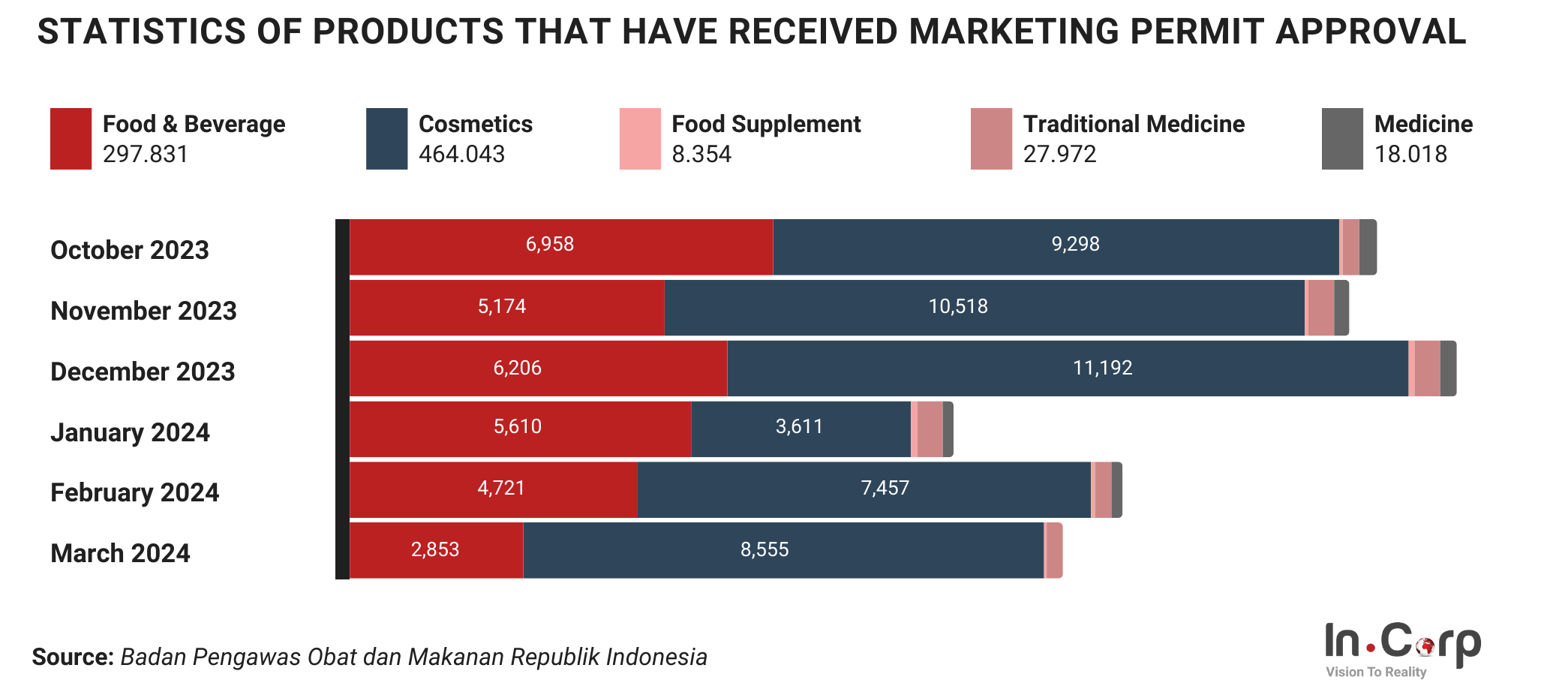
In general, Badan Pengawas Obat dan Makanan (BPOM) has functions similar to those of the Food and Drug Administration (FDA) in the United States (US). The main functions of BPOM in Indonesia include:
- Developing new policies, standards, procedures, and criteria.
- Updating existing policies, standards, procedures, and measures.
- Implementing and overseeing the enforcement of policies, standards, practices, and actions.
- Conducting product laboratory testing.
- Inspecting manufacturing and distribution facilities, as well as product sampling.
- Issuing certificates and permits.
Read more: The halal industry: trends and prospects in Indonesia
The purpose of the BPOM certification
The certification issued by BPOM in Indonesia serves several important purposes. However, the most important certificate is to ensure product safety and quality before being released into the market.
Adherence to regulation
Obtaining BPOM certification is a crucial step in penetrating Indonesia’s market. It is designed to guarantee that the products being sold are safe, high-quality, and meet the strict regulations set by the Indonesian authorities.
Product standardization
The certification provided by BPOM in Indonesia is crucial in promoting standardization and consistency across various product categories. It helps establish a unified framework that fosters reliability and encourages businesses to maintain high-quality standards, ultimately benefiting consumers.
Good manufacturing practices
Manufacturers adhere to strict GMP protocols throughout production, from sourcing raw materials to final product packaging. This effectively reduces the risk of contamination or adulteration.
Product evaluation
The BPOM certification process involves a comprehensive product assessment, including random spot checks, sampling, and laboratory testing. This process identifies potential risks and ensures that the product complies with regulatory standards.
Inspection of manufacturing facilities
The role of BPOM in Indonesia cannot be overstated when it comes to ensuring that manufacturing and distribution facilities comply with regulatory requirements. By performing inspections, BPOM plays a vital role in promoting safety and quality in producing and distributing goods.
Research for policy development
Beyond its regulatory functions, BPOM in Indonesia also conducts research to advance public health.
Who needs to register products for BPOM certification in Indonesia?
Local companies, agents, or distributors are eligible to register food products. However, alternative options exist if you do not have a company or have yet to select an importer.
One option is establishing a foreign-owned company, PT PMA, which allows for 100% foreign ownership. However, the percentage may vary based on specific conditions, such as the sector of business operation.
Foreigners can fully own a PT PMA for importing and exporting goods, but product distribution still requires a local distributor to be appointed. If your business plan includes the distribution of food products, foreign ownership is limited to 67%, necessitating a local partner.
Types of products that must be registered in Indonesia
To have a smooth process of registering your product, it’s important to be familiar with the different types of product registration. Here are a few types you should keep in mind:
Health supplements
Health supplements in Indonesia are formulated to meet individuals’ nutritional needs and enhance their overall health and well-being. These supplements contain various ingredients, including vitamins, minerals, amino acids, and other essential nutrients.
They are typically categorized into three groups based on their ingredients and how they are prepared.
- Category 1: Comprising isolated materials.
- Category 2: Including natural materials.
- Category 3: Involving new forms of preparation, dosages, and applications.
The registration procedures for health supplements closely mirror those for food and beverages, ensuring that products are safe and compliant with regulations.
Medical devices and equipment
In Indonesia, medical devices are classified into four categories, taking into consideration their potential impact on health. The registration process involves a few essential steps that need to be followed.
- Register the legal entity with the Ministry of Health online.
- Conduct inspections of local manufacturers if applicable.
- Register products after determining their class, which permits their distribution and sale.
Cosmetic product registration
Cosmetic products in Indonesia must be registered for each variation, with strict regulations governing labeling, advertising, claim requirements, and ingredient usage. Successful registration requires compliance with these requirements, and the process involves several steps:
- Register the legal entity online with BPOM.
- Complete online registration of the manufacturer.
- Register products, thereby enabling their distribution and sale in the Indonesian market.
BPOM certification codes in Indonesia
BPOM certification employs three distinct codes to differentiate registered products in the market.
- ML (Makanan Luar): This code is specific to imported processed food and beverage products.
- MD (Makanan Produksi Dalam Negeri): This code is specific to domestically produced food and beverage products that must adhere to food safety requirements.
- SP (Sertifikat Penyuluhan): This code is specific to small and medium-sized enterprises (SMEs) receiving assistance from municipal health agencies.
The procedure for obtaining SKI through BPOM registration
It’s important to comply with safety standards set by the Indonesian National Agency of Drug and Food Control (BPOM) when importing medicines, traditional remedies, cosmetics, supplements, and processed foods into Indonesia.
Regulation No. 12 of 2015 governs this process, mandating registration before customs clearance. Follow the outlined steps to obtain a Letter of Import (SKI) from the Head of BPOM:
Step 1: Register online
Go to the BPOM or the INSW website (Indonesia National Single Window) and create your account using Single Sign-On.
Step 2: Gather your documents
Here’s what you’ll need to upload:
- Application letter
- Letter of responsibility
- Importer Identification Number (API) – NIB
- Trading Business License (SIUP)
- Tax ID (NPWP)
- Power of attorney letter for importing (if applicable)
- List of HS codes for the product
- Pharmaceutical Industry License (for drug SKI)
Step 3: Payment
Complete the e-payment for Non-Tax Revenue (PNBP).
Step 4: Submit electronic documents
Now, upload these additional documents:
- Distribution approval license
- Certificate of analysis
- Invoice
- Proof of Payment of Non-Tax Revenue (PNBP)
Extra requirements (depending on your product)
- Vaccines and serums: Batch/lot release certificate, batch/lot protocol summary, and a letter from the producer.
- Processed food: Letter of recommendation (may be required).
What can InCorp Indonesia help with your BPOM certification
Obtaining certification for your product registration in Indonesia can be complex and time-consuming. InCorp Indonesia is your expert throughout this intricate procedure, ensuring compliance with Indonesian regulations.
We specialize in streamlining the registration process for:
Contact our experienced team below for assistance.
Get in touch with us.
What you'll get
A prompt response to your inquiry
Knowledge for doing business from local experts
Ongoing support for your business
Disclaimer
The information is provided by PT. Cekindo Business International (“InCorp Indonesia/ we”) for general purpose only and we make no representations or warranties of any kind.
We do not act as an authorized government or non-government provider for official documents and services, which is issued by the Government of the Republic of Indonesia or its appointed officials. We do not promote any official government document or services of the Government of the Republic of Indonesia, including but not limited to, business identifiers, health and welfare assistance programs and benefits, unclaimed tax rebate, electronic travel visa and authorization, passports in this website.



